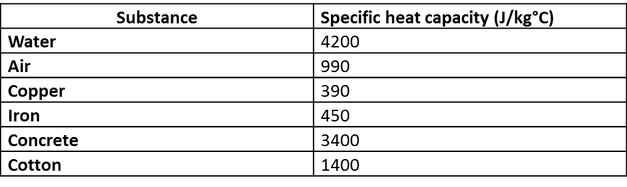
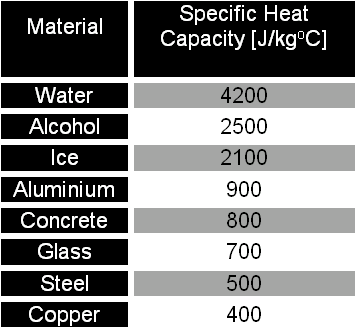
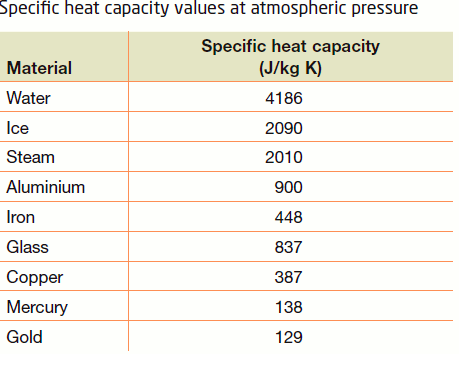
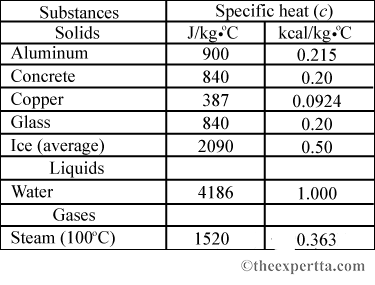
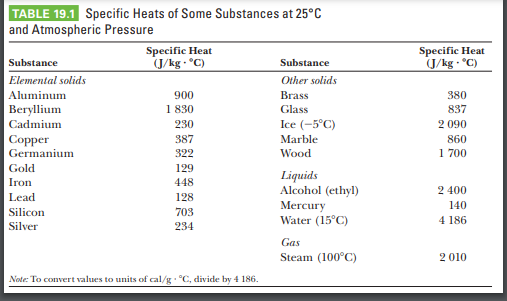
10 g of ice at 0^∘C absorbs 5460 J of heat energy to melt and change to water at 50^∘C . Calculate the specific latent heat of fusion of ice. Specific heat

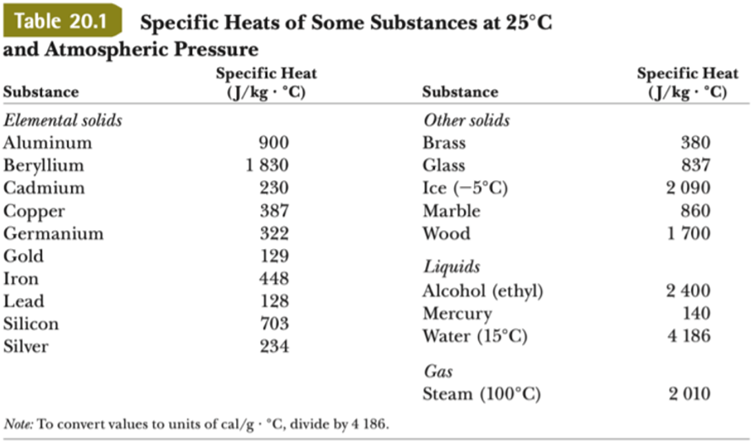
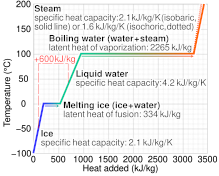
SOLVED: Specific heat capacity of water =4186J kg 1K-1 Specific heat capacity of ice =2090 J kg 1K-1 Latent heat of fusion of ice 335 kJ kg-1 Latent Heat of vaporization of

Learning Outcomes: Rearranging equation for Specific Heat Capacity Topic Equation for Specific Heat Capacity Target Audience: G & T Teacher instructions. - ppt download