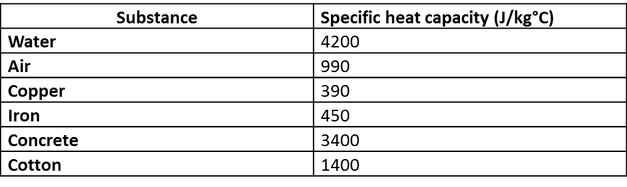
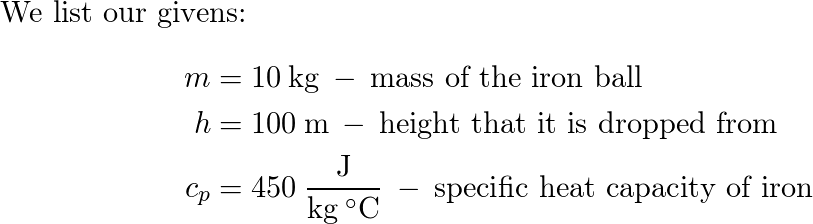1. Calculate the amount of heat required to change the temperature of an iron ball of mass 3 kg from 30° C - Brainly.in

📐PSYW - Please Show Your Work A radiator made out of iron of specific heat capacity 450 J/kgk has a - Brainly.com

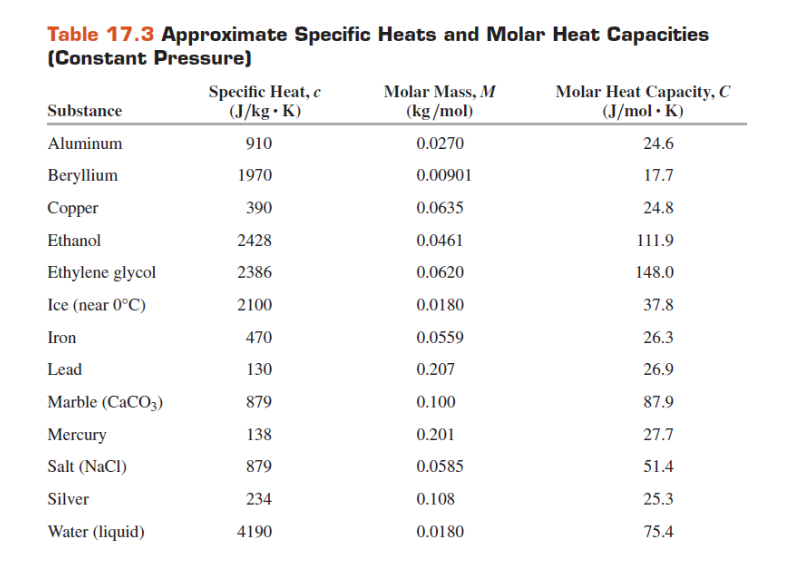
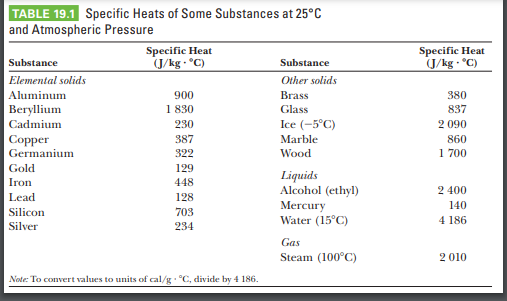
SOLVED: (IV) The specific heat capacity of aluminium is CAt 900 J /(kg-C) , while that of iron is CFe 450 J/(kg-C). (a) Five kilograms of aluminium at 20€ and 10kg ol
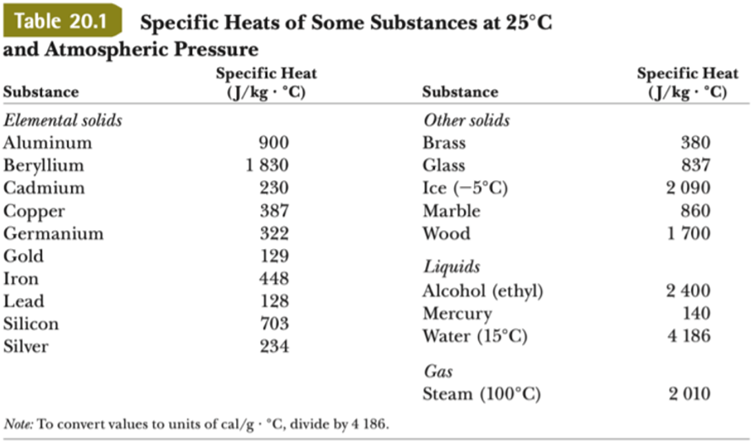
1 Dr.Ali Abadi Chapter Seven: Thermal Properties Materials Properties Heat capacity is a material's ability to absorb
Cheat calculations Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

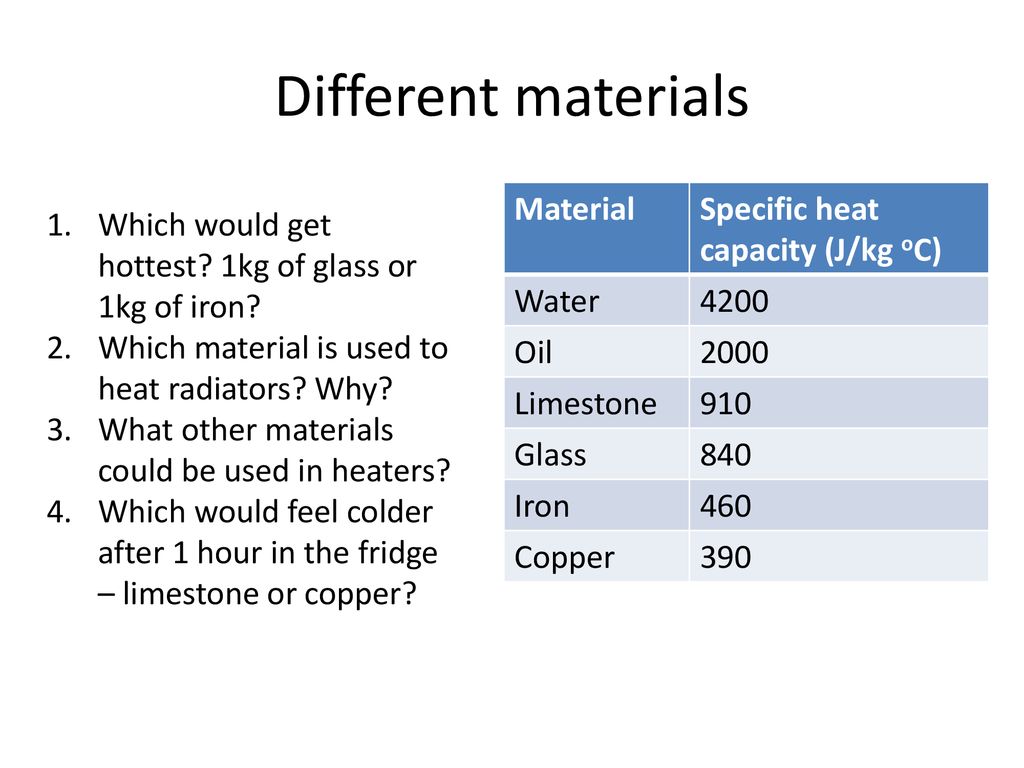
Learning Outcomes: Rearranging equation for Specific Heat Capacity Topic Equation for Specific Heat Capacity Target Audience: G & T Teacher instructions. - ppt download