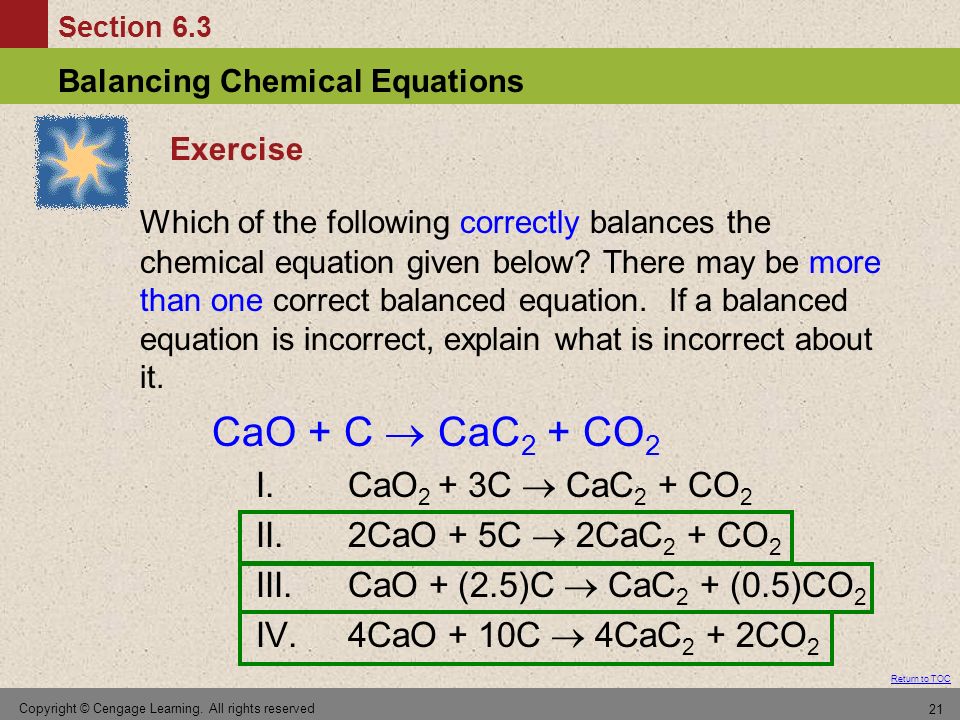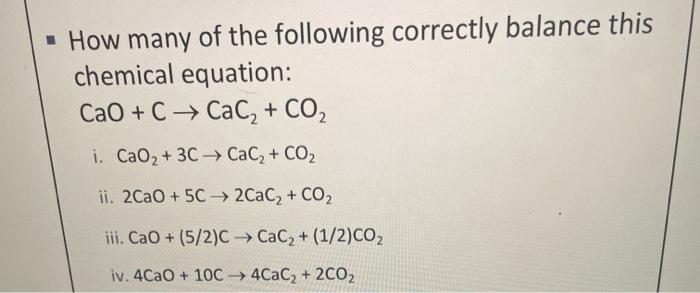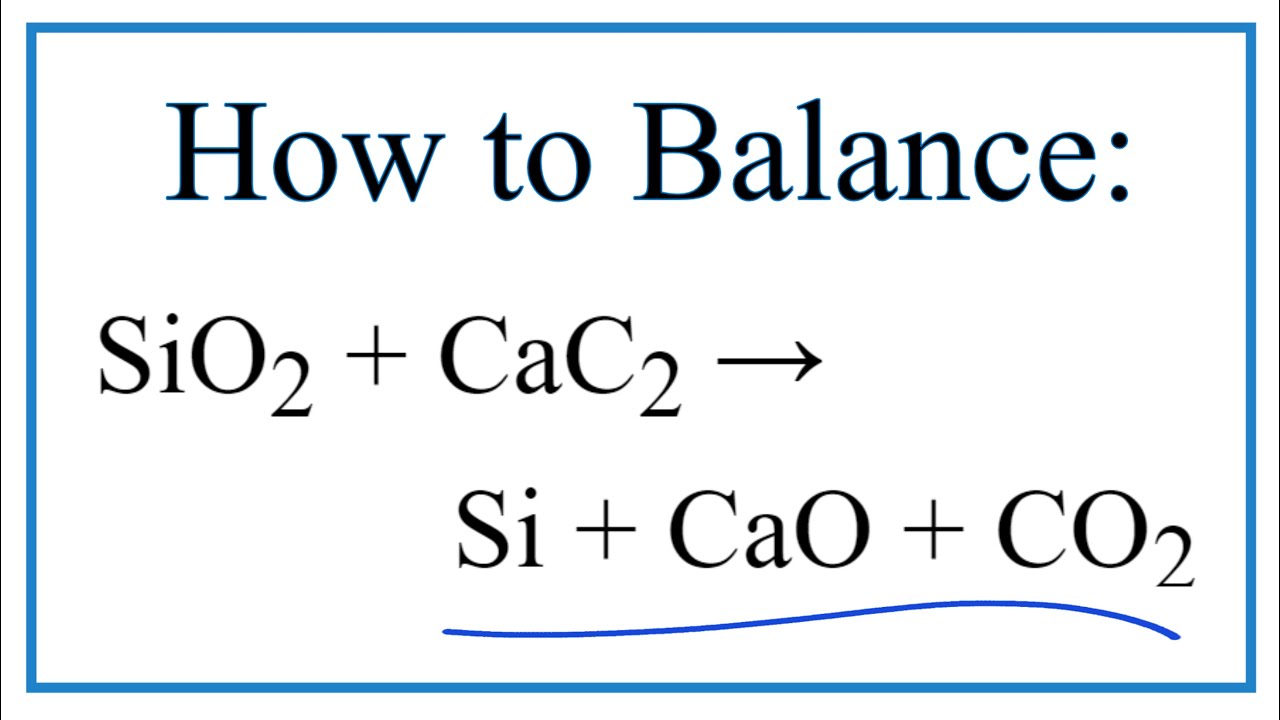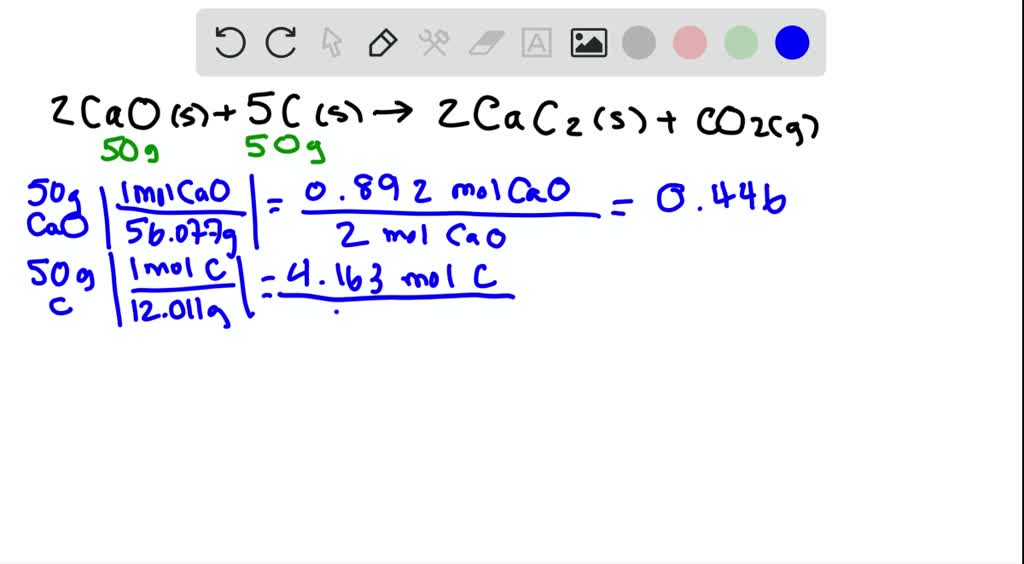
SOLVED:Determine the limiting reactant and mass of left-over reactant when a 50.0 g sample of CaO is reacted with 50.0 g of carbon according to the balanced equation 2 CaO(s)+5 C(s) →2

Chemical Reactions Follow the matter… 1. Chemical Reactions Two chemicals have interacted in some way so that a new substance or substances are formed. - ppt download
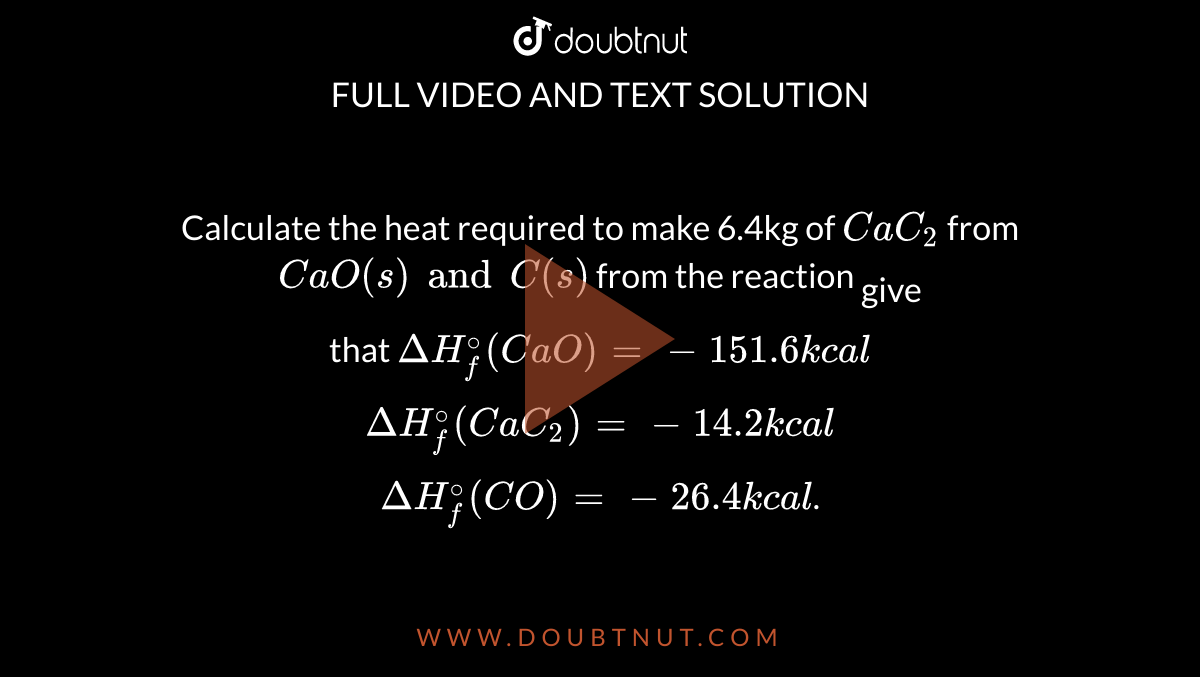
Calculate the heat required to make 6.4kg of CaC2 from CaO(s) and C(s) from the reaction that DeltaHf^@(CaO) = -151.6 kcal DeltaHf^@(CaC2) = -14.2 kcal DeltaHf^@(CO) = -26.4 kcal.

Refer to the following potential energy diagram and the choices above,What is the H of the reaction to form CO from C + O2

Chemical Equation A representation of a chemical reaction: C 2 H 5 OH + 3O 2 2CO 2 + 3H 2 O reactants products. - ppt download
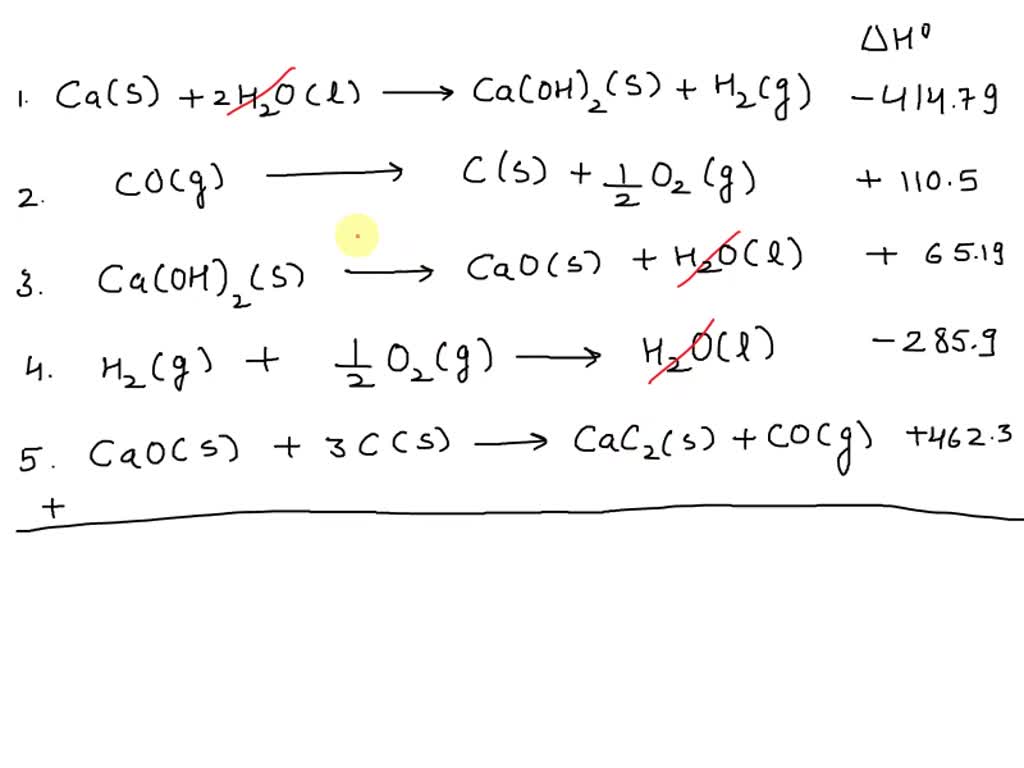
SOLVED: Calculate the standard heat of formation of calcium carbide, CaC2(s), in kJ/mol using the following thermochemical equations. Ca(s) +2H2O(l) 🡪 Ca(OH)2(s) +H2(g) ∆H° = - 414.79 kJ 2C(s) +O2(g) 🡪 2CO(g)
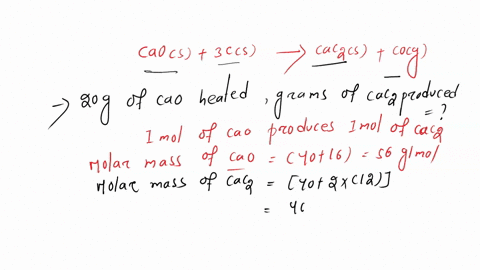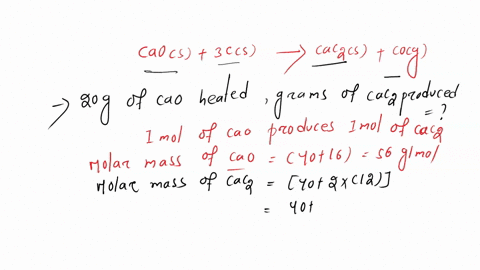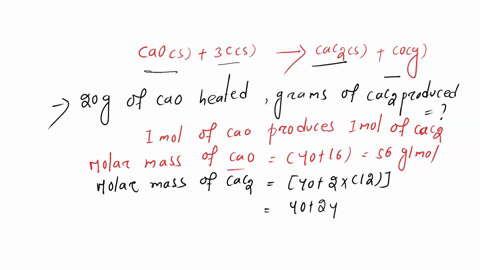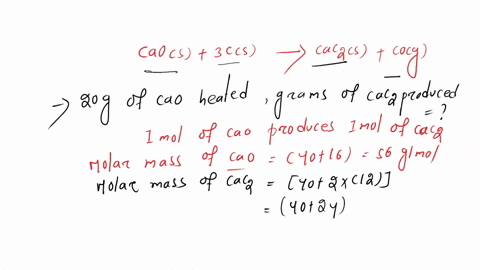
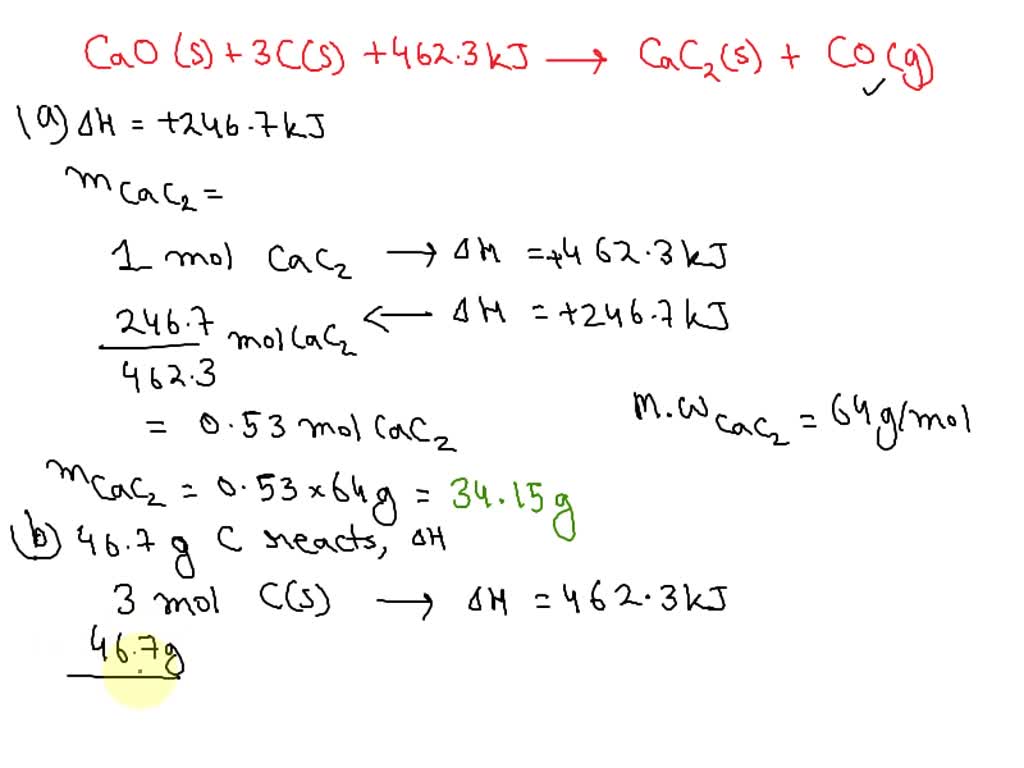
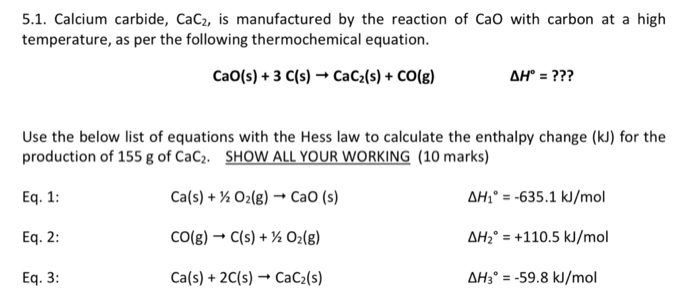
SOLVED: Calcium carbide, CaC2, can be prepared at high temperature by the following reaction: CaO(s) + C(s) -> CaC2(s) + CO(g) a) Balance the chemical equation. b) If a starting mixture contains
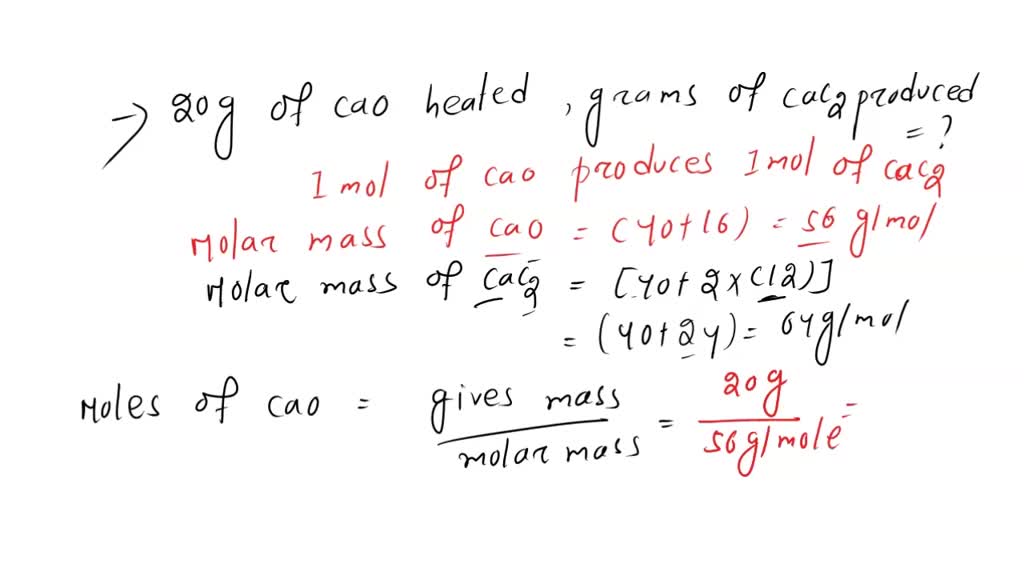
SOLVED: Calcium carbide, CaC2, used to produce acetylene, C2H2, is prepared by heating calcium oxide, CaO, and carbon, C, to high temperature. CaO(s)+ C(s) Cac2(s)+ CO (g) Balance the chemical equation for
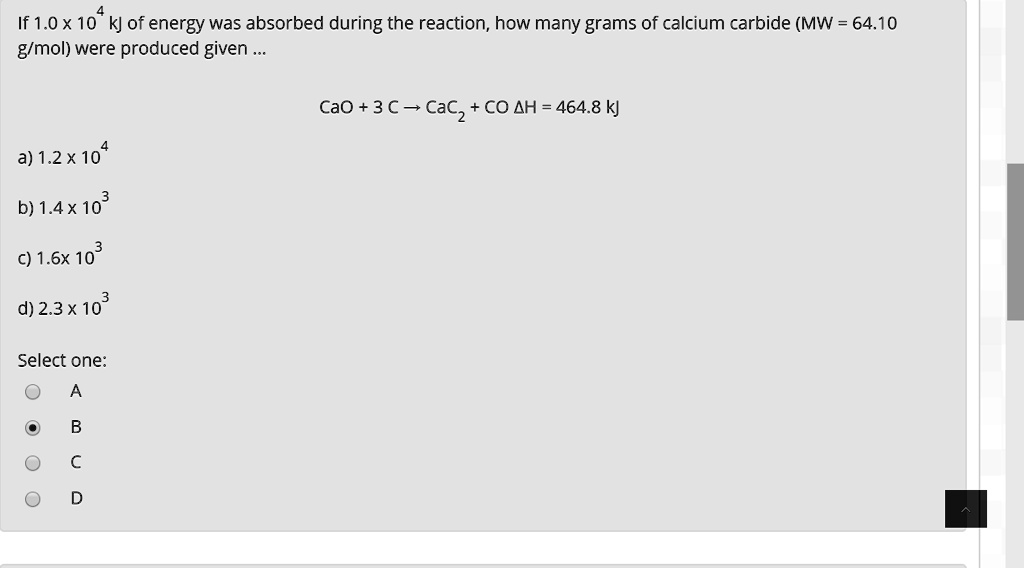
SOLVED: If 1.0X 10 kJ of energy was absorbed during the reaction, how many grams of calcium carbide (MW = 64.10 glmol) were produced given Cao + 3 € CaC2 + CO
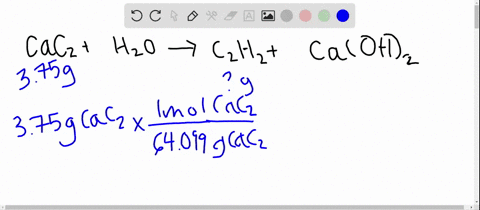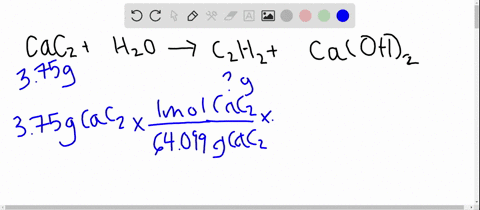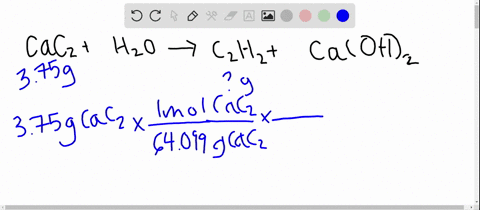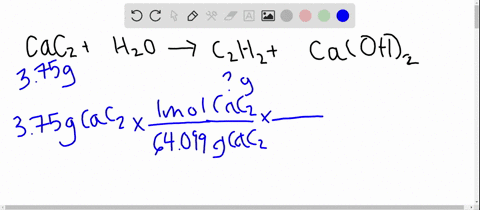
SOLVED: Calcium carbide, CaC2, used to produce acetylene, C2H2, is prepared by heating calcium oxide, CaO, and carbon, C, to high temperature. CaO(s)+ C(s) Cac2(s)+ CO (g) Balance the chemical equation for

From the following reactions at 298 K .(A) CaC2(s) + 2H2O(l) → Ca(OH)2(s) + C2H2 (g); Δ H^∘ = - 127.9 kJ mol^-1 (B) Ca(s) + 12 O2(g) → CaO(s) ; Δ